How to Calculate Neutron Number
D 2 x r Zr1 93 mm to calculate the hydraulic diameter the pitch of fuel pins is. To calculate the total number of present electrons you simply add the amount of extra charge to the atomic number.
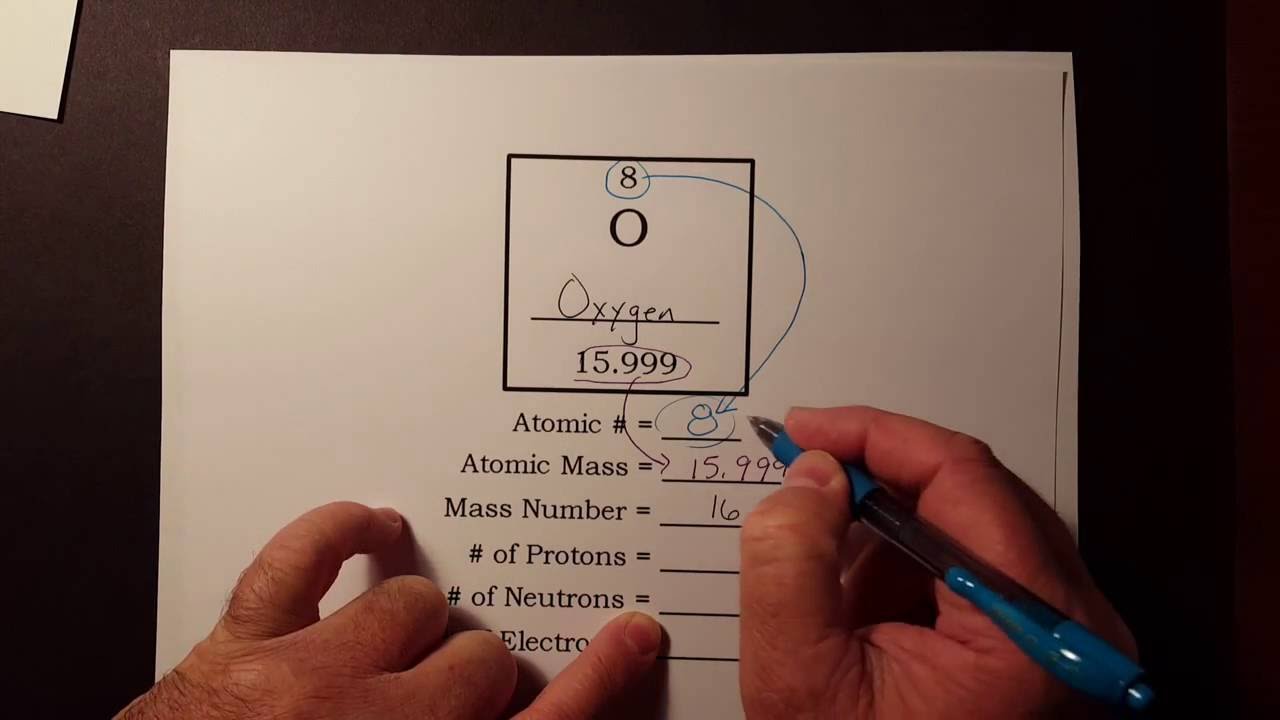
How To Find The Number Of Protons Neutrons And Electrons From The Periodic Table Youtube Neutrons Protons Proton Neutron Electron
Binding Energy formula is defined as the amount of energy required to separate a particle from a system of particles or to disperse all the particles of the system is calculated using Binding Energy Atomic Number Mass-pMass Number-Atomic Number Mass-n-Mass of atomc2To calculate Binding Energy you need Atomic Number Z Mass Number A.

. P 13 mm to calculate the hydraulic diameter the dynamic viscosity of saturated water at 300C is. You can click on the images of the planets to get more information about them from Bill Arnetts incredible Nine Planets web site. When an ion has a negative charge the atom has gained electrons.
A modification in the number of neutrons in the nucleus indicates an isotope. VENUS Your weight is. When we calculate the theoretical mass of a nucleus using the masses of protons and neutrons and compare it to the experimental mass we observe that there is a small but relevant difference in the two masses.
In order for them to be atoms of the same element their number of protons would be the same Examples. ENTER YOUR WEIGHT HERE The Planets MERCURY Your weight is. Calculation of the Reynolds number.
The video describes roughly what happens without exactly following the evolutionary sequence of any one supernova progenitor. For the free neutron the decay energy for this process based on the rest masses of the neutron proton and electron is 0782 343 MeV. Hypernova and Newborn Neutron Star.
That is the difference between the rest mass of the neutron and the sum of the rest masses of the products. Scroll down the page for examples and solutions. Notice that your weight is different on the different worlds.
When converted into kilograms the mass of the neutron can be approximated to 167410-27 kg. The mass of a neutron is 16 x 10 -24 g. The maximal energy of the beta decay electron in the process wherein the.
That difference has to be carried away as kinetic energy. Notice that the weights on other worlds will automatically fill in. A major source of heat loss from a house is through walls.
To calculate the Reynolds number we have to know. It is consistent with being formed in the core-collapse of a single CO star or in. The following diagrams show the isotopes of chlorine and how to calculate the relative atomic mass.
Isotopes are atoms of the same element that have different number of neutrons. The recognition of the element appears the same as the number of neutrons inside the nucleus varies. Thermal neutron with mass of 167 x 10-27 kg travels at a speed of 2200 ms.
Therefore neutrons are neutrally charged subatomic particles. GRB 171205A is a low-luminosity long-duration gamma-ray burst GRB associated with SN 2017iuk a broad-line type Ic supernova SN. There might be zero one two more neutrons in the nuclei depending on the element.
The outer diameter of the cladding is. Assume that the indoor and the outdoor temperatures are 22C and. μ 00000859 Nsm 2.
Suppose you have a chlorine 35 and 37. Click on the Calculate button. In the case of a negative ion there are fewer protons than electrons.
Formula to calculate relative abundance. M2 is the mass of the second isotope. Add the charge to the atomic number for negative ions.
Calculate the rate of heat flux through a wall 3 m x 10 m in area A 30 m 2The wall is 15 cm thick L 1 and it is made of bricks with the thermal conductivity of k 1 10 WmK poor thermal insulator. It is a neutral particle. Calculate the de A.
Isotopes are variants of a particular chemical element which differ in neutron number and consequently in nucleon number. In nuclear engineering a neutron moderator is a medium that reduces the speed of fast neutrons ideally without capturing any leaving them as thermal neutrons with only minimal thermal kinetic energyThese thermal neutrons are immensely more susceptible than fast neutrons to propagate a nuclear chain reaction of uranium-235 or other fissile isotope by colliding with. De brogkie wavelength is given as the the plancks constant divided by product of momentum mv of.
The subatomic particle neutron is present in an atoms nucleusn represents neutron. M1 is the mass of one isotope. James Chadwick discovered the neutron.
ME is the atomic mass of the element from the periodic table. He used scattered particle to calculate the mass of the neutral particle. Hydrogen has three isotopes.
Example Heat Loss through a Wall. The electric charge that is associated with a neutron is 0. The mass of a neutron is roughly equal to 1008 atomic mass units.
The exact sequence and extent of radial growth mass loss and core contraction and the number of these phases will be largely determined by the initial mass of the star and the stellar models used to calculate their evolution. The discovery of neutron didnt happen until the year 1932. This is a result from the binding energy which is responsible for binding protons and neutrons together in the nucleus of an atom.
The nucleus of protium 11H or simply. X is the relative abundance.

3 Ways To Calculate Atomic Mass Wikihow Teaching Chemistry Atoms And Molecules For Kids Chemistry Worksheets

Definition Of Mass Number With Examples Isoptopes Mass Number Definition Of Mass Atomic Mass Unit

Isotope Atoms With The Same Number Of Protons Atomic Number But Different Atomic Masses Chemistry Education Teaching Science Chemistry Labs

Counting Atoms Protons Neutrons Electrons Worksheet Counting Atoms Text Features Worksheet Worksheets
No comments for "How to Calculate Neutron Number"
Post a Comment